Bcs Class 1 Drugs
Generic Name Trade Name BCS Class. 1 abacavir sulfate Ziagen 3 2 acetaminophen Children's Tylenol 3,4 3 acetaminophen/codeine acetaminophen/codeine 4/3.
- Recent FDA Guidance For Industry; BCS Class 1 and 3 August 2015 Bryan Crist Scientific Affairs Manager. (BCS) class 1 and 3 drug substances in. Dosage Forms Containing Biopharmaceutics Classification System Class 1 and 3 Drugs; August, 2015.
- BCS and Dosage Form Trends. It is commonly recognized that most new drugs present formulation challenges. In fact, older drugs as compared to newer ones have higher solubilities in general. One reference noted that BCS Class II compounds as a percentage of compounds under development had increased from 30% to 60%.
Abstract
The biopharmaceutical classification system (BCS) classifies compounds based on their solubility and permeability. Regulatory agencies and health organizations have utilized this classification system to allow dissolution to be used to establish bioequivalence for highly soluble and highly permeable compounds. The pharmaceutical industry has taken advantage of this and BCS-based waivers are becoming more routine and result in significant savings. Further, there is strong scientific rationale to allow BCS-based waivers for even more compounds to realize even more savings. Yet just as clear as the benefits are the barriers that limit application: lack of international regulatory harmonization, uncertainty in regulatory approval, and organizational barriers within the pharmaceutical industry. Once these barriers are overcome and additional applications are fully allowed, the full benefits of BCS applications will be realized.
INTRODUCTION
Amidon et al. first proposed a biopharmaceutic classification system (BCS) in 1995, that classified compounds based on their solubility and permeability (). Subsequently, regulatory agencies and health organizations have utilized this classification system to allow in vitro dissolution to be used to establish bioequivalence for highly soluble and highly permeable compounds (). The pharmaceutical industry has taken advantage of dissolution and BCS-based waivers of in vivo studies; however, this is not the only instance where application of the BCS is beneficial. Rather, its principles are used throughout development. A series of case studies are presented to illustrate uses of BCS through out the clinical development cycle followed by a discussion of its implementation in that aspect of clinical development.
CASE STUDIES I-II—APPLICATION OF BCS IN INNOVATOR DRUG DEVELOPMENT
Case Study I—Use of the BCS in Formulation Development
Pregabalin (Lyrica®) is described chemically as (S)-3-(aminomethyl)-5-methylhexanoic acid. It binds with high affinity to the alpha2-delta site (an auxiliary subunit of voltage-gated calcium channels) in central nervous system tissues. Although the mechanism of action of pregabalin is unknown, results with genetically modified mice and with compounds structurally related to pregabalin (such as gabapentin) suggest that binding to the alpha2-delta subunit may be involved in pregabalin’s antinociceptive and antiseizure effects in animal models. In vitro, pregabalin reduces the calcium dependent release of several neurotransmitters, possibly by modulation of calcium channel function. Pregabalin is indicated for the management of neuropathic pain associated with diabetic peripheral neuropathy, management of postherpetic neuralgia, adjunctive therapy for adult patients with partial onset seizures, and management of fibromyalgia (3).
Pregabalin is a BCS Class 1 compound (highly permeable and highly soluble). Pregabalin is an amino acid and its lowest aqueous solubility occurs at its isoelectric point (at pH 7.4). It is considered high solubility as the amount of water needed (<10 mL) to dissolve the highest dose strength (300 mg) at pH 7.4 is less than the 250 mL criteria. Pregabalin meets the BCS criteria for a highly permeable compound as greater than 90% of the dose is excreted unchanged in the urine (4).
Three different series of formulations were used during clinical development. Each series was comprised of one to three different dose strengths. Strengths within each series were content proportional with respect to drug and excipients. All three series used the same excipients; however the relative proportion of each excipient was different for each series. Bioequivalence between and among these formulations and the commercial formulation was established for this BCS Class 1 compound by demonstrating that all formulations were rapidly dissolving and had similar dissolution profiles over a pH range of 1.2 to 6.8. Thus bioequivalence was demonstrated using dissolution data and waivers of in vivo bioequivalence studies were granted (4,5).
Discussion—Use of the BCS in Formulation Development
Application of BCS had significant impact on the cost of clinical development program for pregabalin. It has been estimated that this example saved the company more than $1,000,000 compared to a more traditional approach that would have utilized four separate bioequivalence studies. Further, indirect savings are equally impressive. Considering that yearly Pregabalin sales exceed $1,200,000,000, each month of times saving equates to an additional $100,000,000 in sales prior to loss of patent exclusivity. Looking across the pharmaceutical industry, approximately 30% of all oral immediate release drugs can be classified as highly permeable and highly soluble (). It has been estimated that the pharmaceutical industry can save over $35,000,000/year in direct savings through application of the BCS (7).
The use of the BCS to obtain a biowaiver of in vivo studies is often the first item that comes to mind when considering how the BCS is used in clinical development. However, the BCS also offers a framework from which to address the assessment of the adequacy of performance of new formulations through out clinical development. Such a strategy is illustrated in Fig. 1.
BCS framework for judging the adequacy of formulation performance
For BCS Class I compounds, dissolution may be used to judge the adequacy of a new formulation. Prior to pivotal efficacy and safety trials, there is no regulatory burden to establish bioequivalency. A company could chose to judge the adequacy of a new formulation using more limited dissolution testing, for example only comparing the new and old formulations at one pH between 1 and 7 where the compound is least soluble. When new formulations are introduced after pivotal efficacy and safety trials have begun, there is a need to establish bioequivalence. This can still be performed with dissolution; however such dissolution testing must conform to regulatory standards.
For BCS Class II compounds, dissolution is the rate limiting step to drug absorption and therefore dissolution can be used to judge the adequacy of performance with the caveat that the dissolution test used should reflect the in vivo performance. In other words it should be possible to develop an in vitro/in vivo correlation (IVIVC). Prior to pivotal efficacy and safety trials, it is possible to develop and use a correlation using a single formulation provided that there is sufficient confidence in the ability of the dissolution method to predict in vivo performance. This correlation should facilitate risk assessment and decision-making within pharmaceutical companies during formulation changes. Once pivotal efficacy and safety trials have started, assessment of new formulations would need to have an IVIVC that is validated to regulatory standards. Lack of an IVIVC would necessitate testing using clinical bioequivalence or bioavailability studies.
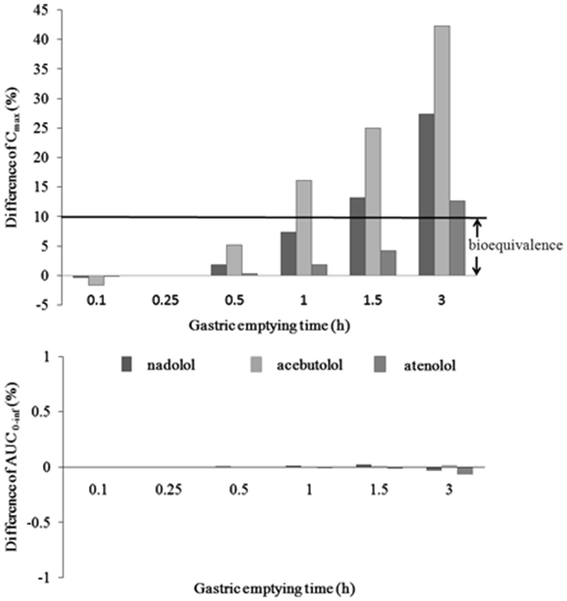
For Class III compounds that are rapidly dissolving one could use dissolution testing to judge adequacy of formulations introduced prior to pivotal efficacy and safety trials. For example, a new formulation may be considered acceptable if both the new and old formulations are more than 85% dissolved in 15 min at pH 1.2 as these conditions assure that the formulations dissolve rapidly in the stomach and that stomach emptying is the rate limiting step in the absorption process. For rapidly dissolving formulations introduced after pivotal efficacy and safety trials as well as non-rapidly (slowly) dissolving formulations, testing will need to conform to regulatory standards. It is worth noting that some agencies do allow dissolution based bioequivalence testing for Class III compounds (8). International relations and diplomacy pdf. Finally for Class IV compounds, clinical bioequivalence and bioavailability studies will need to be performed.
Case Study II—A Case for Regulatory and Industrial Reform
Compound A is a development candidate currently being evaluated in late phase clinical studies. Its solubility is much greater than 1 mg/mL in aqueous medium in the pH range of 1.0 to 7.5. The amount of water needed to dissolve the highest dose strength is less than 2 mL. Based on the current guidance, Compound A is classified as a high solubility compound. Although the in vitro permeability (2.0 × 10−6 cm/s) based on the Caco-2 data was not considered high using metoprolol as the high permeability marker (16 × 10−6 cm/s), Compound A did yield an absolute oral bioavailability of more than 90% in human studies. Hence, Compound A is also classified as a high permeability compound. In addition, Compound A exhibits fast absorption with an early Tmax of <1 h in human with a solution formulation.
During the late phase development, the clinical formulation was changed from a tablet to a capsule formulation. Even though nearly complete release of Compound A was achieved at 30 min timepoint in 0.1 N HCl solution and in pH 4.5 and 6.8 buffers, noticeable differences in initial dissolution rate were observed among the capsule, tablet, and over-encapsulated (OE) tablet formulations with the latter being the slowest (Fig. 2). Specifically, both tablet and OE tablet formulation showed less than 85% drug release at 15 min. F2 values were less than 50 for various comparisons among the three formulations within the 30 min window. Among the three dose strengths, as expected, the dissolution rate difference was the greatest with the highest dose strength. Based on the current FDA guidance (9) for biowaiver using BCS, F2 values of >50 are required for biowaiver of in vivo studies if the drug release is less than 85% at 15 min at physiological pHs. Further, since BCS-based biowaiver approach is not employed to the same extent in EU and Japan in order to allow product filing around world simultaneously, biowaivers of in vivo studies for the three formulations were not pursued.
Differences in rate of dissolution among tablets, encapsulated tablets, and capsules of Compound A. The dissolution tests were carried out as described in the FDA guidance using USP Apparatus I at 100 rpm in three media (data in 10 mM phosphate buffer pH 6.8 shown above)
To assess the potential impact of very rapid dissolution of the new capsule formulation on pharmacokinetic (PK) profiles (especially the Cmax due to very early Tmax) of Compound A, a probe bioavailability (BA) study was conducted to compare the three formulations. The variabilities of pharmacokinetic parameters were assessed in this three-period crossover study with 18 healthy subjects. The dose tested was the highest clinical strength where the greatest differences in dissolution rate were observed. The geometric mean ratios (GMR) at 90% CI for AUC were very close to unity using the OE tablet as the reference formulation (which showed slowest initial dissolution). For the GMR for Cmax, the table formulation met the 0.8–1.25 target while the capsule formulation yielded a range of 0.96–1.27. A trend of higher Cmax value for the capsule formulation was detected in this study. Careful examination of the data showed that high Cmax value from a single subject received the capsule was the primary cause of the failed bioequivalence (BE). In addition, shorter Tmax values were detected for the tablet and the capsule formulation compared to the OE tablets. This probe study indeed confirm that for drugs with early Tmax the PK parameters are more sensitive to formulation changes and higher inter-subject variability can be expected.
Since OE tablet formulation had been used in pivotal efficacy studies, a subsequent definitive BE study for the OE tablet and capsule formulations was conducted. Given the observed difference and variability from the probe BA study, 90 subjects were employed in a two-period crossover design to have a 90% chance of declaring bioequivalence. In contrast to the results from the probe BA study, the two formulations were shown to be bioequivalent with geometric mean ratios for AUC and Cmax achieved nearly unity for this BCS class I compound. It should be noted that a trend of early Tmax for the capsule formulation was confirmed in the definitive BE study but this difference did not lead to bioinequivalence.
Since BE was achieved between the capsule and OE tablet at the highest dose strength despite the greatest difference in dissolution rate, it is expected that lower potency capsules should be bioequivalence to lower potency OE tablets. Based on the results from the definitive bioequivalence study conducted at the highest strength and the proportionally similar excipients used in all three potencies of the same type of formulation (10), waiver of additional BE studies for the two lower strengths was requested to FDA.
Discussion—A Need for Regulatory and Industrial Reform
As previously cited, approximately 30% of all oral immediate release drugs can be classified as highly permeable and highly soluble (). Thus it may be surprising that between 2003 and 2006, only 25 requests (11 for NCEs and 14 for ANDAs) submitted to the FDA for either a BCS classification determination or for a waiver of an in vivo study (12). The perceived lack of certainty of acceptance by the regulatory agency has been cited as one reason for the reluctance to apply for biowaivers (). This is in contrast with the traditional method of using in vivo studies to demonstrate bioequivalence. Pharmaceutical companies know how to run the studies and there is a historical record of regulatory acceptance that provides a sense of certainty. [It should be noted that only one of 11 applications for NCE for BCS classification was turned down by the US Food and Drug Administration (seven requests were granted; more information was requested by FDA for three other applications) (12).
As highlighted in the case of Compound A, there are regulatory barriers that prevent making biowaiver requests routine resulting in the conductance of expensive and time-consuming clinical BA/BE studies. The lack of uniformed employment of BCS-based biowaivers among US and EU/Japan agencies presents a major risk for WMA filing of a new product. Receiving a BCS-based biowaiver from the FDA due to formulation changes in late phase clinical studies may not assure a timely WMA filing. Even though agencies such as the FDA have made significant efforts in promoting the use of BCS-based biowaivers, the time required from filing a biowaiver request to receiving a formal approval from the FDA needs to be fast enough (e.g. within weeks rather than months) to the current uncertainty surrounding its approval when a pharmaceutical company decides to move a development program forward using a BCS-based waiver. Failure to receiving a biowaiver can lead to a significant delay in a development program. Hence, the risk associated with receiving a biowaiver approval often seems greater than benefit of saving resources.
A case may also be made that current guidances contain unnecessary barriers. The case study with Compound A clearly highlights the unnecessary requirement in the current BCS-based biowaiver guidance for F2 values of >50 if the formulations containing BCS class I drugs fail to release more than 85% of drugs in 15 min. The case with Compound A demonstrated that in the extreme scenario where larger differences in initial dissolution rate are detected and drugs have very early Tmax values BE are achieved for formulations that show >85% drug release in 30 min. However, given the pharmacokinetic characteristics of the compound, the differences in dissolution rates were not expected to result in bioinequivalent products (). Hence, difference in dissolution profiles in the first 30 min may not be bio-relevant for rapidly dissolving formulations of BCS class I drugs.
Yet another barrier to biowaiver requests is the compartmentalization of company resources. The cost savings to an organization resulting from a biowaiver typically appears in the budget of the clinical department. However, departments such as preclinical pharmacokinetics, chemistry and formulation may be asked to perform more than the “normal” amount of work to support a biowaiver request. As a result there can be reluctance for all parts of the organization to support a biowaiver strategy. Further, for the compartmentalized large pharmaceutical companies, the reluctance for employing BCS-based biowaiver can also be caused by unclear responsibility for generating the biowaiver documents and accountability if a biowaiver request is rejected and program timeline is delayed.
For the reasons listed above, traditional BE approach of employing clinical studies is still often practiced as the preferred conservative option to ensure development timeline and to address regulatory uncertainty from various agencies around the world. Thus the full benefits of biowaivers to the pharmaceutical industry may not be fully achieved until pharmaceutical companies adopt models that better resource the support of biowaiver requests and regulatory agencies more fully align and create more timely processes.
CASE STUDIES III–V—APPLICATION OF BCS IN GENERIC DRUG DEVELOPMENT
Bcs Class 1 Drugs
Case Study III—Generic Drug Development of a Higher Strength Formulation
The first case study involved the request for the waiver of a bioequivalency study for a higher strength of a product for which a bioequivalency study had previously been submitted to the FDA. The reference product in this case was a tablet having three dosage strengths. The two lower strengths had previously been submitted to FDA based on a fasting and fed bioequivalency study utilizing the intermediate strength. Since literature suggested that the drug is highly soluble and highly permeable, a BCS Class I-based waiver of a fasting bio study for the high strength was desired. Confirmatory solubility studies showed that the drug is highly soluble per BCS criteria, and the reference product was shown to be rapidly dissolving. A rat perfusion study was then performed comparing test compound with a high permeability internal standard, and this study confirmed that the drug has high permeability. A stable dosage form having rapid dissolution was developed, and the ANDA for the higher strength was approved using the BCS approach.
Case Study IV—Generic Drug Development of a Generic Formulation Using Data Generated In-house
The second case study dealt with the submission of an ANDA containing a BCS based bioequivalency waiver request for a product for which the molecule was established as a BCS Class 1 compound through in-house experimentation. In this case the reference product was a tablet having one dosage strength, and the FDA recommendation was to conduct a fasting and food study to establish bioequivalence between test and reference products. Although the product labeling stated bioequivalence between an oral solution and the tablet, there was no reference in the label as to percent of oral dose absorbed. However, a literature search revealed that greater than 90% of an orally administered dose is absorbed. Solubility experiments showed the drug to be highly soluble per BCS criteria, and the reference product shown to be rapidly dissolving. Based on this information, a permeability study was performed via the Caco-2 model, and the compound was shown to be highly permeable by comparison to a highly permeable internal standard. As this information was being generated a stable and rapidly dissolving generic product was developed, and an ANDA was submitted with a request for a BSC biowaiver that was subsequently granted by the FDA.
Case Study V—Generic Drug Development of a Generic Formulation Using Literature Data
The final generic pharmaceutical case study involved the submission of an ANDA for a compound in which the literature clearly established the compound to be BCS Class 1. In this case, solubility and permeability work was performed to confirm literature findings in support of the ANDA submission. The reference product was a tablet with one dosage strength, and labeling for reference product stated that absolute oral bioavailability is approximately 100%. In addition, multiple literature references supported the fact that the drug is essentially completely absorbed after oral administration. Solubility experiments performed in-house showed the drug to be highly soluble per BCS criteria, and the reference product was shown to be rapidly dissolving. Based on this information it was decided to conduct a supportive permeability study, and the compound was shown to be highly permeable via the rat perfusion model by comparison to a highly permeable internal standard. A stable and rapidly dissolving generic product was developed, and an ANDA was submitted with a request for a BCS biowaiver which is currently under consideration by the FDA.
Discussion—Generic Drug Development
The establishment of the BCS and issuance of an FDA guidance document governing the use of the BCS to obtain waivers of bioequivalency studies for immediate-release solid oral dosage forms has made it possible for generic pharmaceutical companies to obtain FDA approval of some generic products without having to conduct a bioequivalency study comparing the generic and brand products. The advent of the BCS has thus made it possible for generic companies to perform drug development on certain products in a more time and cost-effective manner.
In order to execute a BCS development project, a generic company must first identify the target compound as a potential BCS Class 1 candidate through scientific information obtained during pre-formulation studies and from literature references. If the existing literature suggests that the compound is highly permeable, the solubility of the compound must then be confirmed through laboratory experiments. If the compound is found to be highly soluble per the FDA criteria for a highly soluble compound, then permeability studies are conducted to determine if the compound is highly permeable, typically through the use of an in situ animal model or the Caco-2 model. Based on these studies, if the compound is found to be highly soluble and highly permeable, the final objective for a BCS Class I ANDA submission is to develop a manufacturable, stable product that is rapidly dissolving.
It should be noted that the FDA Guidance for Industry allows for the use of a variety of methods to establish high permeability of a compound, including the use of the rat perfusion model as well as the Caco-2 model (9). It has been found that either method is suitable for use in establishing the permeability class of a compound; therefore both methods have been employed during the development of the products presented in Cases III–V. The use of a particular method was predicated upon a variety of factors, including prior literature information regarding the use of a particular method to determine permeability of the compound, development timelines, and availability of resources at outside contract research organizations to perform the studies.
The three case studies noted above demonstrate that the BCS can be strategically deployed to save time and resources during generic drug development. From a financial perspective, the avoidance of a bioequivalence study can be expected to save a generic company several hundred thousand dollars in development costs. In addition, use of the BCS can eliminate the need to expose human subjects to the test and reference products. From an overall timeline perspective there can be advantages to using the BCS approach assuming that required solubility, permeability and dissolution studies are performed in parallel with formulation development. If these studies are executed early in the development process, then several months of time can be shaved off of the overall development timeline. However, this approach should be selectively utilized, carefully considering regulatory risks versus benefit for each project considered for development. In particular, for compounds for which no published permeability literature exists, the risk of a potential rejection by the FDA of a submitted permeability study or a potential delay due to questions that may arise during review of the data must be weighed against the time and cost required to perform a human bioequivalency study. In these situations a case may be made that given the high probability of regulatory acceptance of a positive bioequivalence study, the use of a traditional approach is preferred in order to achieve a timely regulatory approval of the drug product.
CONCLUSIONS
The case studies presented clearly demonstrate that BCS-based biowaivers are becoming more routine and result in significant savings. The possibility also exists to expand application to realize even more savings. Yet just as clear as the benefits are the barriers that limit application: lack of international regulatory harmonization, uncertainty in regulatory approval, and organizational barriers within the pharmaceutical industry. Once these barriers are overcome and additional applications are fully allowed, the full benefits BCS applications will be realized.
References
Abstract
The Biopharmaceutics Classification System (BCS) categorizes drugs into one of four biopharmaceutical classes according to their water solubility and membrane permeability characteristics and broadly allows the prediction of the rate-limiting step in the intestinal absorption process following oral administration. Since its introduction in 1995, the BCS has generated remarkable impact on the global pharmaceutical sciences arena, in drug discovery, development, and regulation, and extensive validation/discussion/extension of the BCS is continuously published in the literature. The BCS has been effectively implanted by drug regulatory agencies around the world in setting bioavailability/bioequivalence standards for immediate-release (IR) oral drug product approval. In this review, we describe the BCS scientific framework and impact on regulatory practice of oral drug products and review the provisional BCS classification of the top drugs on the global market. The Biopharmaceutical Drug Disposition Classification System and its association with the BCS are discussed as well. One notable finding of the provisional BCS classification is that the clinical performance of the majority of approved IR oral drug products essential for human health can be assured with an in vitro dissolution test, rather than empirical in vivo human studies. Where to buy overwatch nerf guns.
INTRODUCTION
The rate and extent of drug absorption from the gastrointestinal (GI) tract are very complex and affected by many factors. These include physicochemical factors (e.g., pKa, solubility, stability, diffusivity, lipophilicity, polar–nonpolar surface area, presence of hydrogen bonding functionalities, particle size, and crystal form), physiological factors (e.g., GI pH, GI blood flow, gastric emptying, small intestinal transit time, colonic transit time, and absorption mechanisms), and factors related to the dosage form (e.g., tablet, capsule, solution, suspension, emulsion, and gel) (1–). Despite this complexity, the work of Amidon et al. () revealed that the fundamental events controlling oral drug absorption are the permeability of the drug through the GI membrane and the solubility/dissolution of the drug dose in the GI milieu. These key parameters are characterized in the Biopharmaceutics Classification System (BCS) by three dimensionless numbers: absorption number (An), dissolution number (Dn), and dose number (D0). These numbers take into account both physicochemical and physiological parameters and are fundamental to the oral absorption process (,). Based on their solubility and intestinal membrane permeability characteristics, drug substances have been classified into one of four categories according to the BCS (Fig. 1). The BCS is one of the most significant prognostic tools created to facilitate oral drug product development in recent years; the validity and broad applicability of the BCS have been the subject of extensive research and discussion (–); it has been adopted by the US Food and Drug Administration (FDA), the European Medicines Agency (EMEA), and the World Health Organization (WHO) for setting bioavailability/bioequivalence (BA/BE) standards for immediate-release (IR) oral drug product approval; and the BCS principles are extensively used by the pharmaceutical industry throughout drug discovery and development (–). In this review, we describe and discuss the impact of the BCS and its scientific basis on regulatory practice of oral drug products and review the provisional BCS classification of the top drugs on the global market. The Biopharmaceutical Drug Disposition Classification System (BDDCS) and its association with the BCS are discussed as well. One important outcome of the provisional classification is that the clinical performance of the majority of approved IR oral drug products essential for human health can be assured with an in vitro dissolution test, rather than empirical in vivo human studies.
The Biopharmaceutics Classification System as defined by Amidon et al. (). The BCS classifies drugs by their solubility and permeability properties in order to stand for the most fundamental view of the drug intestinal absorption process following oral administration
BCS IN REGULATORY PRACTICE
Throughout the past decade, the BCS has become an increasingly important tool in drug product regulation worldwide, by presenting a new paradigm in bioequivalence. Bioequivalence (BE) is the critical step that connects the physical drug product with the clinical properties claimed on its label, ensuring continuing quality of the innovative products and the generic products. Before the presentation of the BCS, the BE standard was solely empirical, depending on in vivo bioavailability (BA) studies, i.e., plasma levels, AUC, and Cmax. By revealing the fundamental parameters dictating the in vivo oral drug absorption process, the BCS is able to ensure BE by mechanistic tools, rather than empirical observation; if two drug products that contain the same active pharmaceutical ingredient (API) have a similar GI concentration–time profile under all luminal conditions, than a similar rate, and extant of absorption is ensured for these products, i.e., they are bioequivalent. Thus, BE can be guaranteed based on in vitro dissolution tests that provide the mechanistic proof for similar bioavailability, rather than empirical in vivo human studies. This is the regulatory waiver of in vivo BE, based on the scientific and mechanistic rationale provided by the BCS. Initially, waivers of in vivo BE were accepted only for Scale-Up and Post Approval Changes (SUPAC), but later, the biowaiver principle was extended to the approval of new generic drug products, thus avoiding unnecessary human experiments and reducing cost and time of developing generic IR oral drug products.
The solubility classification of a given drug is based on the highest dose strength in an IR product. According to the current FDA guidance (18,), drug substance is considered highly soluble if the highest strength is soluble in 250 ml or less of aqueous media throughout the pH range of 1.2–6.8 (the volume of 250 ml is derived from typical BE study protocols that prescribe administration of a drug product to fasting human volunteers with a glass (about 8 oz) of water). Otherwise, the drug substance is considered poorly soluble. A drug substance is considered highly permeable if the extent of intestinal absorption is determined to be 90% or higher. Otherwise, the drug substance is considered poorly permeable. The permeability classification is based either directly on the extent of intestinal absorption of a drug substance in humans determined by mass balance or in comparison to an intravenous reference dose, or indirectly on the measurements of the rate of mass transfer across the human intestinal membrane. Alternatively, animal or in vitro models that predict human intestinal absorption, e.g., intestinal rat perfusion models or epithelial cell culture models, can be used. An IR product is characterized as rapidly dissolved if not less than 85% of the labeled drug amount is dissolved within 30 min using USP Apparatus I at 100 rpm or USP Apparatus II at 50 rpm in a volume of 900 ml or less of each of the following media: (1) acidic media, such as USP-simulated gastric fluid without enzymes; (2) pH 4.5 buffer; and (3) pH 6.8 buffer or USP-simulated intestinal fluid without enzymes. Otherwise, the drug product is considered to be slow dissolving.
Up to now, The FDA has implemented the BCS system to allow waiver of in vivo BA/BE testing of IR solid dosage forms for class I, high-solubility, high-permeability drugs. As for class III (high-solubility low-permeability) drugs, as long as the drug product does not contain agents and/or excipients that may modify intestinal membrane permeability, in vitro dissolution test can ensure BE. The absorption of a class III drug is likely limited by its permeability, less dependent upon its formulation, and its bioavailability may be determined by its in vivo permeability pattern. If the in vitro dissolution of a class III drug product is rapid under all physiological pH conditions, its in vivo behavior will essentially be similar to oral solution (controlled by gastric emptying), and as long as the drug product does not contain permeability modifying agents (this potential effect is largely mitigated by the large gastric dilution), in vitro dissolution test can ensure BE. Hence, biowaivers for BCS class III drugs are scientifically justified and have been recommended (–).
PROVISIONAL BCS CLASSIFICATION OF THE TOP DRUGS
Since its introduction in 1995, the validity and broad applicability of the BCS have been the subject of extensive research and discussion, including an effort to draw a BCS classification of many drug products. In this section, we will review the information gathered in the literature on the BCS classification of the top IR oral drug products on the global market. The majority of the data is based on secondary aqueous solubility references and permeability estimations based on correlations with Log P and CLogP. As such, the classifications are provisional and can be revised as more experimental data become available. Also, it should be well recognized that more extensive solubility, dissolution, and permeability determinations would need to be carried out in order to officially classify these drugs in accordance with current BCS criteria, especially to support a biowaiver application. In addition, the BDDCS and its association with the BCS will be discussed as well.
BCS Classification Based on Literature Data
In order to determine the broad applicability and significance of the BCS, we developed a provisional classification of first the WHO Essential Medicines List () and then extended this analysis to the top 200 drugs on the United States, Great Britain, Spain, and Japan lists (). Values for drug solubility were obtained from standard references (e.g., Merck Index, USP etc.), and the maximum dose strengths were readily available in the list being classified, enabling the calculation of the dimensionless dose number (D0). D0 is the ratio of drug concentration in the administered volume (250 ml) to the saturation solubility of the drug in water (), that may also be viewed as the number of glasses of water required to dissolve the drug dose. A dose number equal or lower than 1 indicated high-solubility, and D0 > 1 signified a low-solubility compound. As for the permeability classification, ideally, this would be based on experimental human jejunal permeability data, or well-defined mass balance studies and/or comparison to an intravenous reference dose. However, since such data is available only for a small number of drugs, the provisional permeability classification was based on correlation of the estimated n-octanol/water partition coefficient using both Final destination 2 free online showbox. Log P and CLogP of the uncharged form of the drug molecule (,). Log P and CLogP values were used for permeability classifications as these parameters are readily attainable for most drugs. The correlations were based on a set of 29 reference drugs for which the actual human jejunal membrane permeability data are available. Drugs exhibiting n-octanol/water partition coefficient value greater than metoprolol (Log P 1.72) were categorized as high-permeability since metoprolol is known to be 95% absorbed from the GI and hence may be used as a reference standard for the low/high class boundary (). One noticeable short coming regarding the permeability prediction by lipophilicity correlations is that drugs whose intestinal absorption is carrier-mediated, either in the absorptive direction or exsorptive direction, will have their permeabilities underestimated or overestimated, respectively.
Since 1977, the WHO has published a list of essential medicines required for basic health care based on public health relevance, efficacy, safety, and cost-effectiveness. A total of 260 drugs are included in the 12th edition of the WHO list from 2002 (31), 123 of which are orally administered drugs. This list classification was subsequently compared with the classification of the top 200 prescribed drugs in the United States that include 141 orally administered drugs (32). Only 43 IR oral drugs appear in both WHO list and top 200 prescribed US drugs, highlighting differences in treatment priorities, social acceptance, and awareness between the US and the developing countries ().
Solubility classification of the drugs on the WHO list and the top 200 US list revealed that 67% and 68%, respectively, are categorized as high-solubility (D0 < 1). This finding was obtained even though a conservative approach was applied for the dose number calculations. A total of 43 and 49 drugs on the WHO list and the top 200 US list, respectively, exhibited solubility lower than 0.1 mg/ml; however, some of these drugs were classified as high-solubility based on the dose number (low dose compounds). This reflects the recent trend towards development of highly lipophilic, but high-potency drugs, leading to low dose that compensate for the poor water solubility (1,33).
Based on Log P or CLogP and permeability correlations, a total of 43% and 50%, respectively, of the WHO list exhibited higher values than the reference drug metoprolol and, hence, were provisionally assigned as high-permeability drugs. For carrier-mediated absorbed drugs, e.g., glucose, l-leucine, phenylalanine, and l-dopa, permeability classification based on partition coefficient (either Log P or CLogP) was false-negative (as expected). Based on Log P correlations, no false-positives were obtained; however, based on CLogP correlations, furosemide and losartan, two low-permeability drugs, were false-positives (). Indeed, both drugs were reported to be susceptible for efflux transport, furosemide by MRP2 (), and losartan by P-gp and potentially MRP2 as well (). Likewise, we have recently found that sulfasalazine is actually a low-permeability drug due to efflux process, even though this drug has Log P and CLogP values higher than metoprolol ().
The percentages of the drugs in IR dosage forms on the WHO list that were classified as class I drugs based on D0 and Log P or CLogP were 23.6% and 28.5%, respectively (Fig. 2). The corresponding percentage of drugs classified as class III drugs were 31.7% and 35.0% (Fig. 2), respectively, and regulatory approval of biowaiver for this class of drugs is scientifically justified and recommended by WHO (36). Hence, the majority of IR oral drug products on the WHO List of Essential Drugs are candidates for waiver of in vivo BE testing based on an in vitro dissolution test. The impact of waiving an expensive in vivo BE testing and its replacement by rapid and affordable in vitro dissolution standards in developing countries is expected to be profoundly significant.
Provisional BCS classification of the 123 oral drugs in immediate-release solid dosage forms on the WHO Essential Medicines List, based on dose number (D0) for the solubility criterion and Log P/CLogP correlations for the permeability classification ()
Similar results were obtained in a subsequent classification of the WHO list of Essential Medicines that was based primarily on human fraction absorbed (Fabs) literature data for the permeability assignment (). Out of 61 drugs that could be reliably classified, 34% were classified as class I, 17% as class II, 39% as class III, and 10% as class IV. In this analysis, hence, more than 70% of the classified drugs proved to be candidates for waiver of in vivo BE testing based on in vitro dissolution test. Of course, other drug product characteristics, such as the therapeutic index and the potential influence of the excipients on the rate and extent of absorption, should also be considered.

In view of the fact that many of the WHO drugs are not on the top 200 drugs lists of the developed countries, a subsequent provisional BCS classification of the orally administered IR solid dosage forms in the top 200 drug products lists from the United States (US), Great Britain (GB), Spain (ES), and Japan (JP) was carried out (). Criteria for solubility/permeability classification were as described above, i.e., D0 calculations based on literature data for solubility and partition coefficients correlation for the permeability. More than 50% of the top 200 drug products on all four lists were oral IR drug products, ranging from 102–113 classified drugs/list. The maximum and minimum dose strengths on the US, ES, and GB were similar, indicating commonality with respect to use and efficacy standards. Conversely, significantly lower doses were found on the JP list compared to the other countries, reflecting differences in therapeutic categories and higher emphasis on safety issues. According to the Japanese Guideline for BE studies, the volume used for D0 calculation is 150 ml; hence, this value was used for the classification of the JP list. A volume of 250 ml was used for the classification of the other three lists ().
The solubility classification of the top selling drugs in the four countries was very similar (~55% high-solubility drugs per list), despite of the fact that only 34–44 drugs on the JP list were in common with the US, GB, and ES lists. Based on D0 and CLogP correlation, the percentage of drugs that were classified as BCS class I drugs were 31%, 30.4%, 30.2%, and 34.5% on the US, GB, ES, and JP, respectively (Fig. 3). The corresponding percentage of drugs classified as class III compounds were 23.0%, 25.8%, 28.0%, and 19.5% on the US, GB, ES, and JP, respectively (Fig. 3). Thus, BE criteria of the majority of the world’s top-selling drugs may potentially be based on a suitable in vitro dissolution test procedure. This information should help pharmaceutical manufacturers to avoid unnecessary human experiments and reduce cost and time of the product development. This is of particular interest in countries with considerably limited health care budget. Hence, BCS contributes to the public health worldwide by significantly enhancing the efficiency in drug development and regulatory approval processes.
Provisional BCS classification of oral drugs in IR solid dosage forms on the top 200 US, GB, ES, and JP drugs lists using dose number (D0) for the solubility criterion and CLogP for the permeability classification ()
It should be noted that the solubility criteria specified in the BCS classification guidelines covers the physiologically relevant pH range (typically pH 1.2, 4.5, and 6.8 buffers). However, the solubility values used in the provisional BCS classifications are based on drug solubility in water only. Thus, for ionizable drugs in which the API solid form is a salt, the value of solubility used for the provisional BCS classification may not be the minimum solubility of the drug over the physiological relevant pH range and could, therefore, represent a best case scenario with regard to aqueous solubility. In fact, 31% of the drugs classified as high-solubility on the WHO list are salts, whereas 36% are free-forms. Likewise, 35–39% of the drugs classified as high-solubility on the US, GB, ES, and JP lists are salts, whereas 16–24% are free-forms.
Provisional BCS Classification Based on In Silico Calculations
It is well recognized that human permeability data are very expensive and difficult to obtain. In addition, at the very early stage of drug discovery and development, very little amount of the API is available for thorough evaluation of BCS classification. Hence, a reliable BCS classification based solely on an in silico approach can be extremely valuable. Certainly, the underlying assumptions and methods used in any computational approach should be carefully evaluated; however, the continuous progress, convenience, and feasibility of in silico methods attract increasing interest.
A set of 185 worldwide IR oral drug products was assigned with provisional BCS classification based on two in silico solubility estimations and three in silico permeability approaches: CLogP (BioLoom 5.0 and ChemDraw 8.0), Log P (MOE Version 2004.03), and KLog P using simplified approach based upon the Crippen fragmentation method that depends strictly on the element type in the molecule (38). An excellent agreement was obtained between the solubility classification based on in silico methods and literature values. The in silico permeability calculations demonstrated ~75% accuracy in classifying 29 reference drugs with human permeability data and ~90% accuracy in classifying the 14 FDA reference drugs for permeability.
The in silico based provisional BCS classification of these 185 drugs showed some interesting trends; for a given solubility classification approach, the BCS classification was not significantly different when different in silico partition coefficient methods were used. The classification by the two solubility approaches for a given partition coefficient method, however, exhibited some systematic differences. The in silico solubility approach underestimated class I and overestimated class II drugs by an identical average of 4.3 ± 1%, while it overestimated class III and underestimated class IV drugs by an identical average of 7.3 ± 0.7%, compared to the classification using reference literature solubility (38). This work suggests that when the in silico method is validated, it is convenient, efficient, and cost-effective in the early preclinical drug discovery setting. Further research should continuously improve the accuracy and reliability of in silico-based BCS classification. Methods for more accurate structure-based prediction of solubility and permeability (e.g., polar surface area) should be further developed and evaluated to enable even more reliable in silico classifications.
The Biopharmaceutics Drug Disposition Classification System
While solubility measurements are relatively easy to carry out and usually there is a broad agreement when classifying drugs as either high- or low-solubility drugs, intestinal permeability is not as routinely measured, particularly using methods and laboratory practice that would allow granting a FDA in vivo biowaiver. Wu and Benet () have noticed that the high-permeability characteristics of BCS class I and II drugs allow ready access to metabolizing enzymes within hepatocytes and suggested that there is a good correlation between the extent of drug metabolism and the permeability as defined in the BCS. This is the BDDCS, claiming that if the major route of elimination of a given drug is metabolism, then the drug is high-permeable and if the major route of elimination is renal and biliary excretion of unchanged drug, then that drug should be classified as low-permeability (). The cutoff was originally set at ≥50% metabolism but later changed to 70% or 90% of an oral dose in human. Additional implications of the BDDCS, e.g., food effect and significance of transporter/enzyme interplay in drug interactions, were suggested as well ().
The key questions, to what extent metabolism can be used as a surrogate for intestinal permeability and under what circumstances drug metabolism may not be viable for permeability predictions, were investigated. A total of 168 drugs were classified by the BDDCS based on solubility and metabolism (). Drugs with ≥50% metabolism were defined as extensively metabolized and thus considered high-permeability drugs. Takagi et al. () compared this BDDCS classification of 164 drugs with the BCS approach using D0 and CLogP. The BDDCS classification indicated that 59 drugs are class I, 51 class II, 42 class III, and 12 drugs out of the 164 are class IV compounds. The BCS classification based on metoprolol as the reference compound indicated a total of 42, 54, 57, and 11 drugs as class I, II, III, and IV, respectively. Hence, excellent agreement between BDDCS and BCS was obtained for the classification of class II and IV drugs but not for class I and III (Fig. 4). It was shown that the differences could be reduced depending on the choice of permeability (fraction absorbed) or percent metabolism dividing line for high/low classification ().
Comparison of the provisional classification of 164 drugs according to the BDDCS and the BCS. BDDCS classification was carried out using 50% as the cutoff for extensive metabolism and the BCS using metoprolol as the reference permeability drug ()
More recently, the extent of metabolism of 51 high-permeability drugs was evaluated (). By using a cutoff of 50% metabolism, 37 drugs (73%) were classified as extensively metabolized and, hence, also high-permeability, according to the BDDCS as well. Hence, 27% of these BCS high-permeability drugs were poorly metabolized compounds, pointing out that high permeability as defined by the BCS does not necessarily dictate extensive metabolism. The authors concluded that the extent of metabolism may be useful in supporting permeability classification only under certain circumstances ().
While permeability classification based solely upon metabolism might fail to correctly classify drugs that are highly absorbed but are excreted unchanged into urine and bile (e.g., amoxicillin, trimethoprim, lomefloxacin, zalcitabine, and chloroquine), lipophilicity considerations alone would not be able to predict active carrier-mediated transport of drugs. Despite these differences, the two approaches indicate that granting a waiver from in vivo BE studies is justified for the majority of drugs (,,).
Additional BCS Classification Sources
In addition to the contributions aiming to provisionally classify different drug lists reviewed so far, several other sources are available as well. Literature search reveals that research articles often offer a BCS classification of the investigated drugs (–). Moreover, starting in 2004, a series of monographs have been published in the Journal of Pharmaceutical Sciences, aiming to evaluate all relevant data available from literature sources for a given API to assess the risk associated with a biowaiver. For this purpose, risk is defined as the probability of an incorrect biowaiver decision, as well as the consequences of an incorrect biowaiver decision in terms of public health and individual patient risks. On the basis of these considerations, a recommendation was made as to whether a biowaiver is advisable or not. The monographs have no formal regulatory status but represent the best scientific opinions now available. So far, about 20 APIs evaluated in these monographs, and an in vivo BE waiver was scientifically justified and recommended for vast majority of them (–). These monographs are part of an ongoing project, and the details and progress of this project are available at www.fip.org/bcs. Additional source for BCS classification can be found in Therapeutic Systems Research Laboratory (TSRL Inc., Ann Arbor, MI) website (http://www.tsrlinc.com/services/bcs/search.cfm). This free service offers a provisional BCS classification of a large database of drugs, including the classification process, e.g., Log P/CLogP, solubility value, maximum/minimum dose strength, and calculated dose number (Fig. 5).
The provisional BCS classification service as offered by Therapeutic Systems Research Laboratory (TSRL Inc., Ann Arbor, MI) website
CONCLUSIONS
In conclusion, the majority of the world’s top-selling drugs may be candidates for waiver of in vivo BE testing based on appropriate in vitro dissolution test. The replacement of expensive in vivo testing with a simpler, more easily implemented, routinely monitored, and more reliable in vitro dissolution test would ensure clinical performance of approved drug products in a rapidly globalizing market. Moreover, the current FDA guidelines on BCS classification are considered highly conservative, especially with respect to the class boundaries of solubility, permeability, and dissolution. New regulatory policies, with criteria and class boundaries that will allow granting an in vivo biowaiver to larger number of drugs, should be constructively examined (1,,,).
From industrial point of view, the information provided by the BCS classification of the top drugs on the global market should help pharmaceutical manufacturers of both new medicines and generic drug products to avoid unnecessary human experiments and reduce cost and time of the product development. With continued industry emphasis on more efficient processes and decreasing drug development timeline, the BCS will remain an invaluable tool in the future as well (,).
References